Currents & Gradients
Two factors govern the flow of ions through open channels in a membrane. One factor, the chemical gradient, has to do
with the actual number of ions positioned at the inner and outer 'mouth' of the pore of an open channel.
The other factor, the electrical driving force, involves electrical attractions and repulsions at these same two locations.
If a positive ion, positioned at a mouth of a pore, is surrounded by other positive ions, the tendency will be for it to be 'repelled'
through the pore.
On the other hand, if that same positive ion is surrounded by negative ions, the tendency will be for it to be 'attracted' toward them
and the probability of it passing through the pore will be reduced.
Working together, both of these factors influence the flow of ions through open channels.
Currents
A current is the directional flow of charges (ions in this case). When a channel is open
ions actually flow in both directions but the flow in one direction is usually greater than the flow in the
other direction. The net flow, whichever the direction, is referred to as the current. For example, if 100 ions
flow in and 25 flow out each millisecond, then the current is the net influx of 75 ions per millisecond. Another
example would be 300 ions flowing out per millisecond and 10 flowing in resulting in a net effluxing current at the
rate of 290 ions/millisecond.
Current is measured in amperes; this
is a measure of the number of charges passing a point each second. One ampere represents more than 6,000 quadrillion
charges (6X1018). But usually microAmps (micro- means millionth (10-6)) or nanoAmps
(nano- means billionth (10-9)) are used to describe the magnitude of a current. It's still a big number.
Concentration Gradients
The concentrations of individual ions in the cytoplasm as well as the extracellular fluids remain fairly
constant. Physiological concentrations are usually expressed in millimoles/liter (mM/L); a millimole is a thousandth
of a mole (1x10-3 X 6.024X1023 = 6.024x1020) ... another large number. Following are the
concentrations for the major ions inside and outside of body cells:
- Potassium (K+): 150 inside/ 4 outside
- Sodium (Na+): 20 inside/ 145 outside
- Calcium (Ca++): 0.0001 inside/ 2.5 outside
The ions that pass through the cell membrane will mix with the intra- and extracellular fluids and the above values
will not change due to the movement of these ions. However, measurements taken at the very inner and/or outer surfaces of membranes will
show the effects of these ion movements.
The difference between inside and outside concentrations of an ion is called the concentration gradient. These values are important in understanding the overall direction of ion movement through open
channels. For example, since there are almost 40X more potassium ions inside the cell than outside, the net flow
of these ions will be from inside to outside. The same reasoning
holds for sodium; the net flow of sodium ions will be inward because there are ~7X as many of them outside
compared to inside. A calcium current will definitely be inward because there are 10,000 more calcium ions outside than inside.
Net ion flow is 'down the concentration gradient' (diffusion) from the region of high toward the region of low concentration.
Electrical Driving Force
Since ions are charged particles they will experience electrical attractions and repulsions.
On the whole, the cytoplasm and extracellular fluids are electrically neutral.
However, this is not the case along the very
inner and outer surfaces of the cell membrane due to 'unbalanced' movement of positive ions in and out of the cell.
Assume there is no charge difference between the two surfaces of the membrane.
If sodium channels open, the overall movement will be inward ... down the sodium ion concentration gradient.
This is a loss of positive charge (Na+) from the outer-membrane surface and a gain of positive
charge for the inner-membrane surface. The result is that now there is a charge difference between the two
surfaces. This difference is called an electrical gradient and it establishes the electrical driving force
that governs the how fast the current flows. As this ion movement continues, the difference in charges will increase with the outer surface becoming
increasingly negative and the inner surface becoming increasingly positive.
However, the continued movement of sodium ions down their concentration gradient will begin slowing
because their concentration gradient is becoming smaller. In addition, the region they are entering is becoming more positive while the
region they are leaving is becoming less positive. An electrical gradient is building. Eventually a point will be reached
at which their movement 'down the concentration gradient' will be counterbalanced by the electrical gradient pulling them in the
opposite direction. When this occurs, there will no longer be a
current because ions will be moving at an equal rate in both directions.
As described in Currents & Gradients, the overall concentration gradients are essentially unaffected by ion movement through membrane channels.
However, the electrical driving force at the membrane surfaces changes constantly due to ion movement through open channels. If positive ions enter
the cell the inner-membrane surface becomes more positive (or less negative) while the opposite change is occurring
at the outer-membrane surface. If positive ions leave the cell the inner-membrane surface becomes less positive (more negative)
while the opposite change is occurring at the outer-membrane surface. The difference in charge between the two
surfaces of the membrane is called an electrical gradient. The greater this gradient the greater will be the electrical
driving force.
Voltage & Measuring the Electrical Gradient
An electrical gradient, or separation of charges, exists within a battery. The greater this charge separation the greater the
'potential' to create a current between it's positive and negative ends if a path exists. This 'potential' driving force can be measured
with a voltmeter whether it exists in a battery or between the two surfaces of a cell membrane.
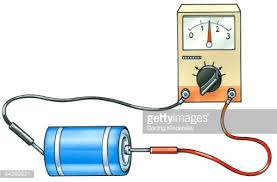 The cell membrane is much like the dividing wall inside a two-cell battery that separates the positive and negative charges. To measure
the extent of this charge separation the electrodes of a voltmeter are held against the exposed metal surfaces of each cell. The illustration
shows a red electrode held to the small nub at one end (the + cell) and the black one to the other end (the - cell). The wire/voltmeter/wire
combination forms a path for negative electrons to travel from the - cell toward the + cell. As this occurs the needle in the voltmeter reflects
the driving force behind this current of electrons. This measure of the electrical driving force is called voltage.
The cell membrane is much like the dividing wall inside a two-cell battery that separates the positive and negative charges. To measure
the extent of this charge separation the electrodes of a voltmeter are held against the exposed metal surfaces of each cell. The illustration
shows a red electrode held to the small nub at one end (the + cell) and the black one to the other end (the - cell). The wire/voltmeter/wire
combination forms a path for negative electrons to travel from the - cell toward the + cell. As this occurs the needle in the voltmeter reflects
the driving force behind this current of electrons. This measure of the electrical driving force is called voltage.
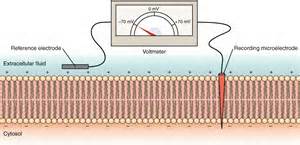 The same technique can be used to measure charge separation across a membrane.
The right illustration shows a membrane with a 'recording' electrode penetrating the cell membrane and a 'reference' electrode held against
its outer surface. The needle on the voltmeter points to -70mV. This indicates the charge 'recorded' at the inner surface is 70mV more
negative than the charge a the outer surface. This membrane has a potential of -70mV.
The same technique can be used to measure charge separation across a membrane.
The right illustration shows a membrane with a 'recording' electrode penetrating the cell membrane and a 'reference' electrode held against
its outer surface. The needle on the voltmeter points to -70mV. This indicates the charge 'recorded' at the inner surface is 70mV more
negative than the charge a the outer surface. This membrane has a potential of -70mV.
Updated:5/2/2015
Continue to Potentials
Return to previous tutorial ... Inactivation
Return to home page
 The cell membrane is much like the dividing wall inside a two-cell battery that separates the positive and negative charges. To measure
the extent of this charge separation the electrodes of a voltmeter are held against the exposed metal surfaces of each cell. The illustration
shows a red electrode held to the small nub at one end (the + cell) and the black one to the other end (the - cell). The wire/voltmeter/wire
combination forms a path for negative electrons to travel from the - cell toward the + cell. As this occurs the needle in the voltmeter reflects
the driving force behind this current of electrons. This measure of the electrical driving force is called voltage.
The cell membrane is much like the dividing wall inside a two-cell battery that separates the positive and negative charges. To measure
the extent of this charge separation the electrodes of a voltmeter are held against the exposed metal surfaces of each cell. The illustration
shows a red electrode held to the small nub at one end (the + cell) and the black one to the other end (the - cell). The wire/voltmeter/wire
combination forms a path for negative electrons to travel from the - cell toward the + cell. As this occurs the needle in the voltmeter reflects
the driving force behind this current of electrons. This measure of the electrical driving force is called voltage. The same technique can be used to measure charge separation across a membrane.
The right illustration shows a membrane with a 'recording' electrode penetrating the cell membrane and a 'reference' electrode held against
its outer surface. The needle on the voltmeter points to -70mV. This indicates the charge 'recorded' at the inner surface is 70mV more
negative than the charge a the outer surface. This membrane has a potential of -70mV.
The same technique can be used to measure charge separation across a membrane.
The right illustration shows a membrane with a 'recording' electrode penetrating the cell membrane and a 'reference' electrode held against
its outer surface. The needle on the voltmeter points to -70mV. This indicates the charge 'recorded' at the inner surface is 70mV more
negative than the charge a the outer surface. This membrane has a potential of -70mV.